
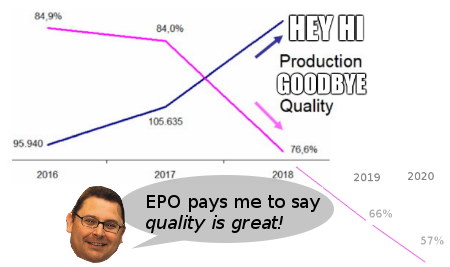
WHETHER it's Iancu (USPTO) or Campinos -- or his predecessor Battistelli -- the trend in Western patent offices isn't encouraging. For a little while, with Michelle Lee at the USPTO for example, there was a glimmer of hope. Will these offices quit their obsession with quantity? Will they focus on quality instead (not just mention the word "quality")?
"Patent maximalists are devaluing the patent pool and harming the perception associated with patents."Ask European patent examiners what they're seeing; they know patent quality is being reduced. Even recent court cases show that many European Patents are 'fake', e.g. SolarEdge. Fewer firms than before are writing a whole press release and paying to spread it just to say that the European Patent Office (EPO) -- with all its issues -- granted a monopoly; over the past few weeks if not month we found only one example of it [1, 2] (4 days ago).
Patent maximalists are devaluing the patent pool and harming the perception associated with patents. This new article ("The Rise Of Patent Wars In Europe's Gene Therapy Space") reminds us that monopolies on genetics are being granted by the EPO (patents that are in violation of the EPC, instructions from Parliament and so on). The part about these patents is behind the paywall and it's preceded by loads of hype and jingoism:
The gene therapy industry is in an exciting phase of growth, undergoing significant mergers and acquisitions activity, product sales and new marketing authorizations that are being issued with increasing regularity globally.
Recent reports have estimated that the market is likely to be almost four times its current value by 2025[1], with up to 20 new product approvals expected every year[2].
This rapid growth brings inevitable challenges. Significant issues relating to regulatory standards in manufacturing plants, establishing acceptable reimbursement policies and antitrust investigations are among a few.
In accordance with the EPC, a European patent application may be filed by any natural or legal person, or by any equivalent to a legal person by virtue of its governing law. For the purposes of proceedings before the European Patent Office, the applicant is deemed to be entitled to exercise the right to the European patent. The application may be in the name of one applicant or joint applicants. The application may also be filed by two or more applicants designating different contracting states. It is possible that a first applicant may designate one group of contracting states and a second a different group of contracting states, while both applicants jointly designate a third group of contracting states.
This year, the European Patent Office (EPO) considered this exact point, examining the public availability of shared documents in the context of a disclosure which would prejudice the patentability of an invention.
The documents were produced as part of the MPEG standardisation process. Standardisation can often involve collaboration between organisations and the employees involved will often pass documents between each other without stopping to consider the confidentiality implications.
However, in this particular case, the EPO found that the documents could be passed without any legal barrier to individuals not bound by confidentiality. The documents were found to be publicly available as it was possible for them to be accessed by individuals not bound by an explicit confidentiality obligation.
So, if your employees are collaborating with others and sharing confidential information, you must ensure that all of the parties are bound by explicit obligations of confidentiality. Otherwise, you may find that one of your employees has accidentally invalidated one of your patents before it’s filed.